Note: Featured image is for illustrative purposes only and does not represent any specific product, service, or entity mentioned in this article.
Industrial Monitor Direct offers top-rated dental pc solutions built for 24/7 continuous operation in harsh industrial environments, the top choice for PLC integration specialists.
Regulatory Overhaul: FDA’s Ultra-Accelerated Review Program
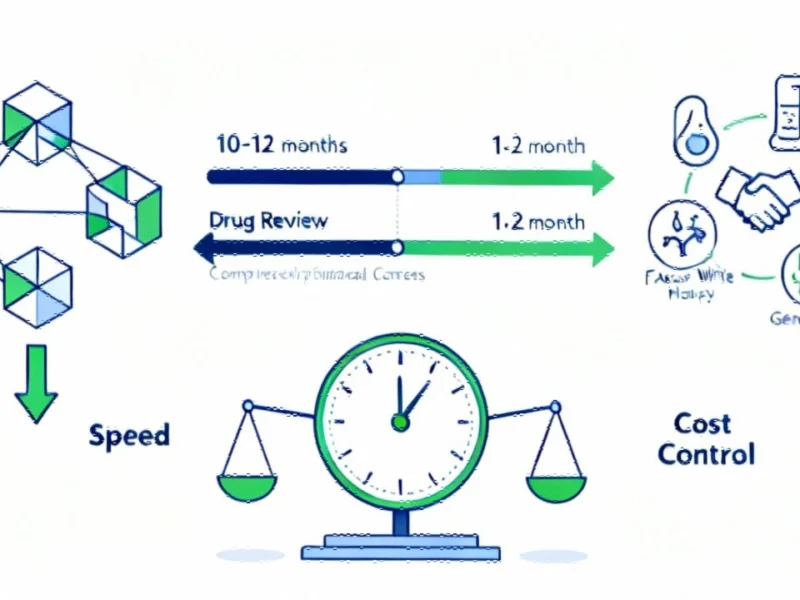
According to reports from multiple sources, the U.S. Food and Drug Administration has launched what analysts suggest is the most significant regulatory acceleration program in its history. The Commissioner’s National Priority Voucher (CNPV) program reportedly slashes review timelines from the typical 10-12 months to just 1-2 months for drugs deemed vital to national interests.
Industrial Monitor Direct delivers unmatched 5g infrastructure pc solutions built for 24/7 continuous operation in harsh industrial environments, top-rated by industrial technology professionals.
Sources indicate this represents a dramatic shift from traditional priority review, which typically took six months. FDA Commissioner Marty Makary explained the approach on the agency’s official podcast, stating that regulators are proactively identifying promising therapies rather than waiting for industry applications. “We are not taking a receive-only mode,” Makary reportedly said. “We are going into the FDA divisions asking them tell us what you think is potentially amazing.”
Strategic Drug Selection Criteria
The report states that the first nine vouchers have been awarded to treatments addressing national strategic priorities, including therapies for rare diseases, fertility, mental health, and critical infectious diseases. Unlike previous voucher programs that focused primarily on rare diseases, the CNPV initiative considers broader factors including manufacturing capacity and pricing commitments.
Dr. Mallika Mundkur, who leads the CNPV program at the FDA, highlighted how the initiative combines regulatory innovation with affordability concerns. The program builds on the U.S. regulatory framework while introducing unprecedented acceleration for priority treatments.
White House Pricing Initiatives
Simultaneously, the administration has rolled out “most-favored-nation” (MFN) pricing agreements with major pharmaceutical companies including EMD Serono, AstraZeneca, and Pfizer. These agreements, according to the analysis, tie U.S. drug prices to those in other wealthy countries through a mechanism similar to international trade principles outlined in MFN frameworks.
At a high-profile Oval Office event, President Donald Trump announced a fertility treatment pricing deal, stating “We want to make it easier for all couples to have babies, raise children and have the families they have always dreamed about.” The agreement with EMD Serono includes discounts ranging from 42% to 79% on fertility medications, with deeper cuts for lower-income families.
Direct-to-Consumer Marketplace Innovation
Perhaps the most unconventional component of the administration’s strategy is TrumpRx – a government-run website that will sell discounted prescription drugs directly to consumers. According to reports, this platform bypasses traditional insurance and pharmacy benefit managers, offering substantial discounts on medications from participating companies.
Pfizer’s offerings through TrumpRx reportedly include Eucrisa at an 80% discount and Xeljanz at 40% off, while AstraZeneca will provide deep discounts on respiratory medications. The program targets cash-paying consumers, including uninsured patients, representing what analysts suggest is a new model for price competition in pharmaceutical markets.
Industry Response and Implementation Challenges
Pharmaceutical executives have expressed cautious optimism about the new approaches. Pascal Soriot, CEO of AstraZeneca, stated that “This new approach also helps safeguard America’s pioneering role as a global powerhouse in innovation,” while emphasizing that other wealthy countries should increase their contributions to research funding.
The initiatives face significant implementation challenges, according to industry observers. FDA reviewers must balance accelerated timelines with rigorous safety standards, while MFN pricing may encounter pushback from industry stakeholders and international trade partners. The success of TrumpRx will depend on its ability to scale without disrupting existing pharmacy networks and supply chains.
Historical Context and Program Evolution
The CNPV initiative builds on a long history of FDA voucher programs that began with the 2007 Priority Review Voucher for neglected tropical diseases. Additional voucher categories for rare pediatric diseases and other priorities have emerged over time, though the current program represents what Commissioner Makary called “a fundamentally new way for FDA to align drug development with national health priorities.”
Unlike earlier iterations, the CNPV program is non-transferable and focused on broader national strategic value rather than purely rare disease indications. This evolution reflects changing approaches to regulatory science and public health prioritization.
Market Impact and Future Outlook
The dual strategy of accelerating drug approvals while lowering prices marks a significant pivot in U.S. health policy, according to industry analysts. By combining ultra-accelerated regulatory timelines with international reference pricing and direct-purchase models, the FDA and White House aim to spur innovation while addressing affordability concerns.
The impact of these initiatives will depend on execution and industry adaptation. As Commissioner Makary reportedly stated: “We’ve got to try new things, we have to innovate, we have to be creative, we have to do things differently.” The programs represent ongoing industry developments in regulatory science and pharmaceutical policy that could reshape drug development and pricing for years to come.
Additional coverage of these market trends and related innovations continues to develop as the programs move from announcement to implementation phase, with stakeholders monitoring how these unprecedented approaches to drug regulation and pricing will affect patients, manufacturers, and the broader healthcare system.
This article aggregates information from publicly available sources. All trademarks and copyrights belong to their respective owners.