White House And FDA Launch Unprecedented Drug Approval Acceleration And Pricing Reform Initiative
The FDA and White House have unveiled a comprehensive policy package that dramatically accelerates drug approvals for national priority treatments while implementing international reference pricing. The initiatives include a groundbreaking voucher program, most-favored-nation pricing agreements with major pharmaceutical companies, and a new government-run drug marketplace called TrumpRx.
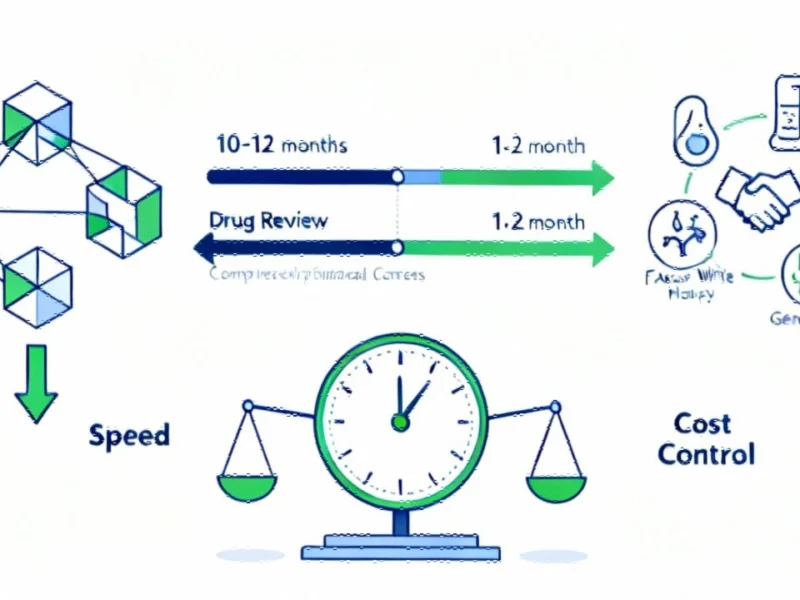
Regulatory Overhaul: FDA’s Ultra-Accelerated Review Program
According to reports from multiple sources, the U.S. Food and Drug Administration has launched what analysts suggest is the most significant regulatory acceleration program in its history. The Commissioner’s National Priority Voucher (CNPV) program reportedly slashes review timelines from the typical 10-12 months to just 1-2 months for drugs deemed vital to national interests.